BY PRINCELY ONYENWE, IMO
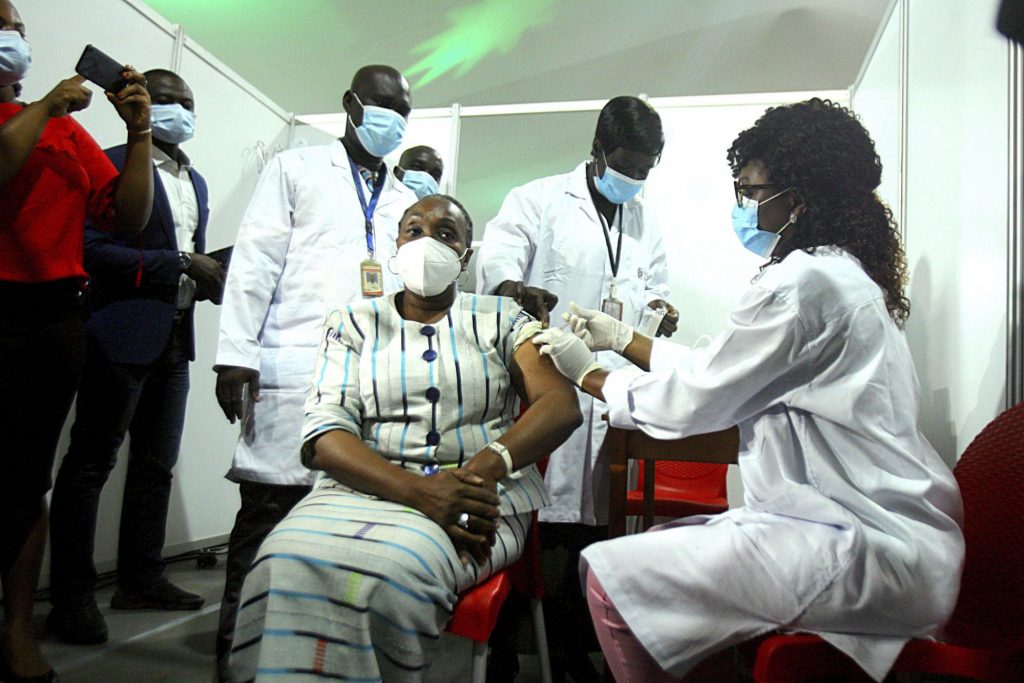
FG has commenced the covid-19 vaccination in the federal capital ABUJA Nigeria.
9News Nigeria reliably Confirmed it’s vaccination process in good order. Health workers in the federal capital territory ABUJA have commenced the national vaccination of covid-19 as approved by the World health organization WHO.
The process commenced with the health workers while the president and vice president has followed suit.
Recall that this covid-19 vaccine was brought to the country few days ago in compliance to the protocols thereto.
The lifting of the covid-19 vaccine will go round to the 36states of the federation through the state governors and the health commissioners from respective states.
Over here in Imo state, His Excellency governor Uzodinma has assured by the arrival of the vaccine today, it’s vaccination will commence by tomorrow 6th march 2021.
The question trailing all over the world is whether the vaccine is real?
WHO has this answer to tell us…”Is there a vaccine for COVID-19?
Yes there are now several vaccines that are in use. The first mass vaccination programme started in early December 2020 and as of and as of 15 February 2021, 175.3 million vaccine doses have been administered. At least 7 different vaccines (3 platforms) have been administered.
WHO issued an Emergency Use Listing (EULs) for the Pfizer COVID-19 vaccine (BNT162b2) on 31 December 2020. On 15 February 2021, WHO issued EULs for two versions of the AstraZeneca/Oxford COVID-19 vaccine, manufactured by the Serum Institute of India and SKBio. WHO is on track to EUL other vaccine products through June.
The products and progress in regulatory review by WHO is provided by WHO and updated regularly. The document is provided here.
Once vaccines are demonstrated to be safe and efficacious, they must be authorized by national regulators, manufactured to exacting standards, and distributed. WHO is working with partners around the world to help coordinate key steps in this process, including to facilitate equitable access to safe and effective COVID-19 vaccines for the billions of people who will need them. More information about COVID-19 vaccine development is available here.
It is expected of the government to give more orientation through relevant agencies to educate the public and make sure the covid-19 vaccination process will not be trauncated by hereasy.
9News Nigeria (Owerri)
For inquiries on this news contact 9News Nigeria Imo State @08036856526