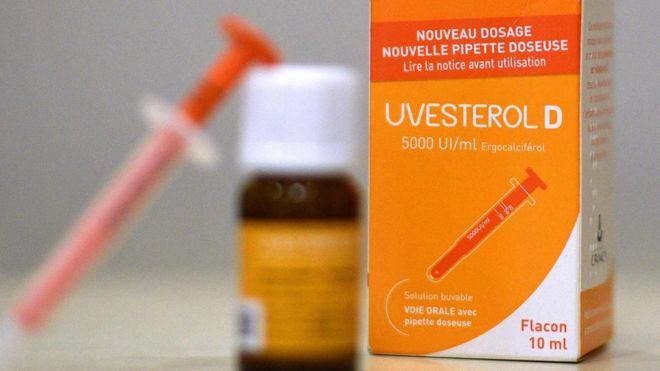
France has acted to suspend the sale of a vitamin D supplement after the death of a newborn baby who suffocated hours after being given it.
The 10-day-old baby had been given a dose of Uvesterol D, widely given to French children under the age of five to prevent vitamin D deficiency.
France’s medical safety agency said there was a “probable link” to that particular supplement.
But officials said there were many other products that could be used.
Health Minister Marisol Touraine said children were not in danger by taking vitamin D supplements in general as “it’s the specific way the product is administered that poses risks”. She promised parents “transparent, objective and reliable information.”
In a statement (in French), the national medical safety agency (ANSM) said “only Uvesterol D administered with a pipette is involved”. The product is not sold in the UK.
The baby died at home on 21 December, apparently after being given a dose of the substance orally through a plastic pipette. It showed immediate signs of suffocation before dying two hours later of cardio-respiratory arrest.
 Image copyrightREUTERS
Image copyrightREUTERSNews of the baby’s death was not disclosed by France’s health authorities immediately but emerged in French media on Monday.
ANSM said that in 2006 it had imposed measures to reduce risks from taking Uvesterol D after adverse effects became known. However, until December there had been no deaths since it went on the market in 1990, it added.
French daily Le Monde has revealed that Uvesterol D has for years been at the centre of fears over how it has been ingested, with several cases documented of serious illness. The paper cited the oily nature of the substance as being different from other types of liquid vitamin D.
The supplement’s producer Crinex changed the pipette in 2006 to prevent the liquid being administered too quickly.
Then, in 2013, the medical safety agency urged parents to give the supplement drip-by-drip before feeding and ensure the baby was in a semi-sitting position. It also reduced the recommended dosage.
In 2014, health journal Prescire called for an end to the use of Uvesterol vitamin supplements for newborn babies, complaining of half-measures and procrastination from both the company and the medical safety agency.

